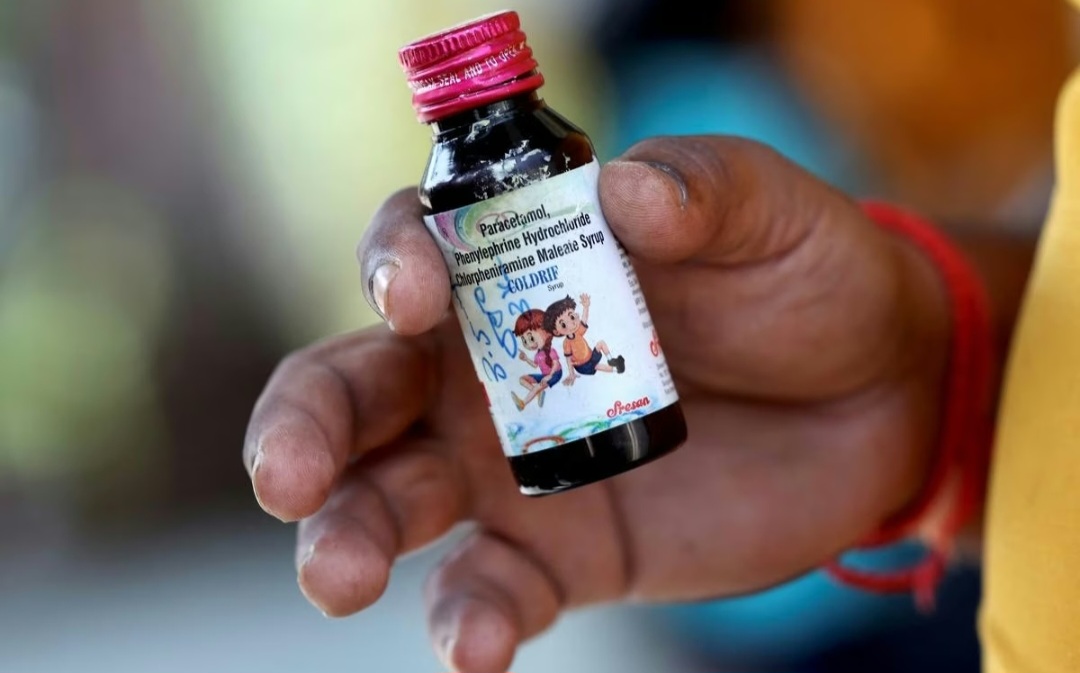
The World Health Organization (WHO) has asked Indian authorities whether the cough syrup Coldrif, implicated in the deaths of at least 22 children, was exported to other countries. The request for clarification comes as the WHO contemplates issuing a Global Medical Products Alert—a warning circulated to member nations about potentially unsafe or contaminated medicines.
The syrup was found to contain harmful substances — diethylene glycol and ethylene glycol — both toxic when present in medications. In Madhya Pradesh, dozens of children have died, while several others remain critically ill with kidney complications.
In response, India’s drug regulator, the Drugs Controller General of India (DCGI), has instructed all state and union territory drug controllers to intensify quality checks on raw materials and finished pharmaceutical products before release into the market. An advisory issued on October 7 noted serious lapses, including manufacturers not testing every batch of excipients or active ingredients to meet required standards.

The Karnataka Assembly Becomes A Battlefield Of Constitutional Authority, As The Governor’s Refusal To Deliver The Government’s Script Triggers A Massive Political Firestorm.

New Evidence From The Sky And The Ground Paints A Damning Picture Of Systemic Failure, Where A Lack Of Simple Signage And A Hesitant Rescue Effort Led To A Fatal Tragedy.

The Elevation Of Nitin Nabin Signals A Bold New Chapter For The BJP, Marrying Grassroots Tradition With A Tech-Forward Vision Led By A President The Prime Minister Now Calls His “Boss.”

Taslima Nasreen’s Critique Reopens The Conversation On Secularism And Celebrity Responsibility, Using The Career Paths Of Bollywood’s Biggest Stars To Challenge The Music Maestro’s Stance.

A Young Life Is Cut Short By A "Planned" City’s Unplanned Dangers, Triggering A Massive Government Probe Into The Safety Lapses That Left A Father Listening To His Son’s Final Pleas.
Karnataka Administration Reaffirms Zero Tolerance For Misconduct As A Senior Police Chief Faces Potential Ouster Over Serious Allegations Of Professional Impropery.

Governor RN Ravi’s Refusal To Deliver The State Address Deepens The Constitutional Crisis In Tamil Nadu, Reaffirming A Total Breakdown In Communication Between Lok Bhavan And The DMK Administration.

The Tragic Death Of Yuvraj Mehta Serves As A Grim Reminder Of The Costs Of Administrative Apathy, Where A Young Life Was Lost In A Preventable Accident Under The Eyes Of Helpless Bystanders.

PM Modi And President MBZ Anchor The India-UAE Strategic Axis Amid Global Volatility, Charting A Shared Course For Regional Peace And Economic Dominance In The 21st Century.

D.Y. Chandrachud Reaffirms That Pre Trial Detention Cannot Be Used As A Punitive Tool, Calling On The Judiciary To Scrutinize Prolonged Incarceration Against The Shield Of Constitutional Liberty.

Authorities Conduct Damage Assessments Across The Himalayan Belt Following A Significant Seismic Event That Has Reawakened Concerns Over The Region’s Geological Vulnerability.

The Mahayuti Alliance Faces Its First Internal Test As Eknath Shinde Leverages His Kingmaker Status To Demand Key Administrative Portfolios In Exchange For Supporting A BJP Mayor.

ED Unveils A Shadow Network At Al Falah University Where Terror-Linked Appointments And Fabricated Medical Records Were Used To Mask A Half Billion Dollar Money Laundering Operation

UP Authorities Decry "Digital Sabotage" Of Varanasi Heritage Project As Police Use Cyber Forensics To Prosecute Those Circulating Fake Architectural Renders Of Manikarnika Ghat

BJP Rejects AR Rahman’s Claims Of Religious Discrimination In The Film Industry As A Base-Less Allegation That Undermines The Merit-Based Culture Of Indian Cinema

Kulman Ghising, credited with ending Nepal’s power cuts and reforming the energy...

NATO forces successfully intercepted a large-scale overnight drone raid launched...

Priya Sachdev’s lawyer has questioned Karisma Kapoor’s absence from Sunjay Kapur...

Qatar has strongly condemned what it describes as a “cowardly criminal assault”...

Karnataka Congress MLA Satish Krishna Sail has been arrested by the Enforcement...